Malaria is a parasitic infection due to protozoa of the genus Plasmodium, transmitted to humans by the bite of Anopheles mosquitoes. Transmission by transfusion of parasite infected blood and transplacental transmission are also possible.
5 species of Plasmodium cause malaria in humans: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. All species may cause uncomplicated malaria. Severe malaria (defined by the presence of complications) is almost always due to P. falciparum. and, less frequently, P. vivax and P. knowlesi.
Uncomplicated malaria can rapidly progress to severe malaria, and severe malaria may cause death within a few hours if left untreated.
Clinical features
Malaria should always be considered in patients living in or coming from, an endemic area, who presents with fever (or history of fever in the previous 48 hours).
Uncomplicated malaria
Fever is frequently associated with chills, sweating, headache, muscular ache, malaise, anorexia or nausea. In children, fever may be associated with abdominal pain, diarrhoea and vomiting. Mild to moderate anaemia is frequent in children and pregnant women.
Severe malaria
In addition to the above, patients presenting with one or more of the following complications
[1]
Citation
1.
World Health Organization. Guidelines for the treatment of malaria, 3rd ed. World Health Organization. 2015.
https://apps.who.int/iris/handle/10665/162441
should be hospitalised immediately:
- Impaired consciousness, including coma.
- Seizures: more than 2 episodes of generalised or focal (e.g. abnormal eye movements) seizures within 24 hours.
- Prostration: extreme weakness; in children: inability to sit or drink/suck.
- Respiratory distress: rapid, laboured breathing or slow, deep breathing.
- Shock: cold extremities, weak or absent pulse, capillary refill time ≥ 3 seconds, cyanosis.
- Jaundice: yellow discolouration of mucosal surfaces of the mouth, conjunctivae and palms.
- Haemoglobinuria: dark red urine.
- Abnormal bleeding: skin (petechiae), conjunctivae, nose, gums; blood in stools.
- Acute renal failure: oliguria (urine output < 12 ml/kg/day in children and < 400 ml/day in adults) despite adequate hydration.
Laboratory
Parasitological diagnosis
[2]
Citation
2.
World Health Organization. Compendium of WHO malaria guidance: prevention, diagnosis, treatment, surveillance and elimination. 2019.
https://apps.who.int/iris/handle/10665/312082
Diagnosis of malaria should be confirmed, whenever possible. If testing is not available, treatment of suspected malaria should not be delayed.
Rapid diagnostic tests (RDTs)
a
Citation
a.
Most rapid tests detect the following antigens alone or in combination: HRP2 protein specific to P. falciparum; an enzyme (Pf pLDH) specific to P. falciparum; an enzyme (pan pLDH) common to all 4 plasmodium species. HRP2 may continue to be detectable for 6 weeks or more after parasite clearance; pLDH remains detectable for several days (up to 2 weeks) after parasite clearance.
Use pan pLDH tests as first choice in hyper and holo-endemic areas, as well as in areas of intense seasonal transmission and during outbreaks or complex emergencies. In other contexts, HRP2 tests (P. falciparum > 95%) or HRP2 + pLDH combination tests (P. falciparum < 95%) are preferred.
Rapid tests detect parasite antigens. They give only a qualitative result (positive or negative) and may remain positive several days or weeks following elimination of parasites.
Microscopy
Thin and thick blood films enable parasite detection, species identification, quantification and monitoring of parasitaemia.
Blood films may be negative due to sequestration of the parasitized erythrocytes in peripheral capillaries in severe malaria, as well as in placental vessels in pregnant women.
Note: even with positive diagnostic results, rule out other causes of fever.
Additional examinations
Haemoglobin (Hb) level
To be measured routinely in all patients with clinical anaemia, and in all patients with severe malaria.
Blood glucose level
To be measured routinely to detect hypoglycaemia in patients with severe malaria and those with malnutrition (see Hypoglycaemia, Chapter 1).
Treatment of malaria due to P. vivax, P. ovale, P. malariae, P. knowlesi
chloroquine (CQ) PO
b
Citation
b.
If the patient vomits within 30 minutes after administration: re-administer the full dose. If the patient vomits between 30 minutes and 1 hour after administration, re-administer half of the dose. If severe vomiting precludes oral therapy, manage as severe malaria, see Treatment of severe malaria.
Children and adults:
Day 1: 10 mg base/kg
Day 2: 10 mg base/kg
Day 3: 5 mg base/kg
In general P. vivax remains sensitive to CQ but resistance is found in several countries. Where such resistance is high (>10%), or in countries which have de-registered CQ due to P. falciparum resistance, an artemisinin-based combination therapy (ACT)
c
Citation
c.
ACT: a combination of artemisinin or one of its derivatives (e.g. artesunate, artemether) with another antimalarial of a different class.
should be used instead
[1]
Citation
1.
World Health Organization. Guidelines for the treatment of malaria, 3rd ed. World Health Organization. 2015.
https://apps.who.int/iris/handle/10665/162441
. For dosing information, see Treatment of uncomplicated falciparum malaria.
Relapses can occur with P. vivax and P. ovale due to activation of dormant parasites in the liver. Primaquine PO for 14 days (0.25 to 0.5 mg/kg once daily in children ≥ 15 kg; 15 mg once daily in adults) can be given to eliminate these parasites, after the initial treatment with CQ or an ACT. However, this treatment is only recommended for patients living in areas where reinfection is unlikely, i.e. non-endemic, low transmission areas or in countries aiming for elimination of malaria. This treatment is contra-indicated in individuals with G6PD deficiency. If G6PD deficiency cannot be tested individually, the decision to prescribe primaquine must take into account the prevalence of deficiency in the population.
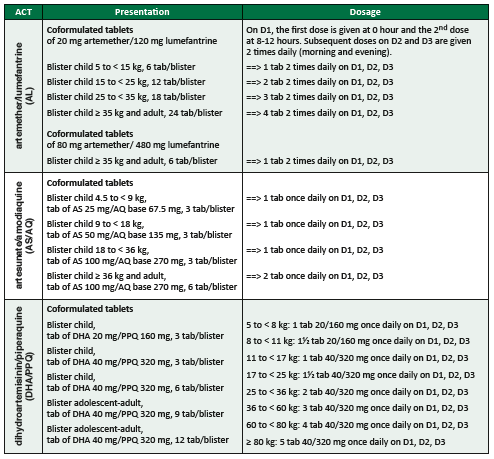
Treatment of uncomplicated falciparum malaria
Antimalarial treatment
During pregnancy, see Antimalarial treatment in pregnant women.
Treatment is an artemisinin-based combination therapy (ACT)
c
Citation
c.
ACT: a combination of artemisinin or one of its derivatives (e.g. artesunate, artemether) with another antimalarial of a different class.
given by the oral route for 3 days
[1]
Citation
1.
World Health Organization. Guidelines for the treatment of malaria, 3rd ed. World Health Organization. 2015.
https://apps.who.int/iris/handle/10665/162441
. The first-line ACT is chosen according to therapeutic efficacy in the area where the patient is living. If the first line ACT is unavailable, contra-indicated or has failed despite being correctly administered, use another ACT. For dosing information, see table below.
Treatment of uncomplicated falciparum malaria
b
Citation
b.
If the patient vomits within 30 minutes after administration: re-administer the full dose. If the patient vomits between 30 minutes and 1 hour after administration, re-administer half of the dose. If severe vomiting precludes oral therapy, manage as severe malaria, see Treatment of severe malaria.
In low malaria endemic areas, in addition to ACT, all individuals (except in children < 30 kg, pregnant women or breastfeeding women of infants aged < 6 months) diagnosed with P. falciparum malaria, should be given a single dose of 0.25 mg/kg primaquine to reduce the risk of transmission
[3]
Citation
3.
World Health Organization. WHO policy brief on single-dose primaquine as gametocytocide in Plasmodium falciparum malaria. 2015.
https://www.who.int/malaria/publications/atoz/who_htm_gmp_2015.1.pdf?ua=1
.
Notes:
- In infants below the age/weight mentioned in the table above, there is little data on efficacy and safety of ACTs.
- The combinations AL, AS/AQ and DHA/PPQ can be used. The dose should be calculated so as to correspond to 10-16 mg/kg/dose of lumefantrine; 10 mg/kg daily of amodiaquine; 20 mg/kg daily of piperaquine.
- Clinical condition of young children can deteriorate rapidly; it may be preferable to start parenteral treatment straight away (see below).
Quinine PO is not recommended as standard treatment, however still remains in some national protocols:
quinine PO for 7 days
b
Citation
b.
If the patient vomits within 30 minutes after administration: re-administer the full dose. If the patient vomits between 30 minutes and 1 hour after administration, re-administer half of the dose. If severe vomiting precludes oral therapy, manage as severe malaria, see Treatment of severe malaria.
Children and adults under 50 kg: 10 mg/kg 3 times daily
Adults 50 kg and over: 600 mg 3 times daily
Symptomatic treatment
Paracetamol PO in the event of high fever only (Fever, Chapter 1).
Treatment of severe malaria
The patient must be hospitalised.
Antimalarial treatment
During pregnancy, see Antimalarial treatment in pregnant women.
Pre-referral treatment
If the patient needs to be transferred, administer before transfer:
- At community level, for children under 6 years: one dose of rectal artesunate
d
Citation
d.
If it is impossible to refer a patient to a center capable of providing parenteral treatment, rectal artesunate should be given according to the same schedule as artesunate slow IV injection (H0, H12, H24, then once daily).
(10 mg/kg)
- Children 2 months to < 3 years (≤ 10 kg): 1 rectal capsule (100 mg)
- Children 3 to < 6 years (≤ 20 kg): 2 rectal capsules (200 mg)
or
- At dispensary level, for children and adults: the first dose of artesunate or, if unavailable, the first dose of artemether. For dosing information, see below.
In either case, provide patients, especially children, with some sugar prior to or during transfer.
Inpatient treatment
The drug of choice is artesunate, preferably IV, or if not possible IM.
For patients in shock: IM route is not appropriate, use artesunate IV only.
artesunate slow IV injection (3 to 5 minutes) or, if not possible, slow IM injection, into the anterior thigh:
Children under 20 kg: 3 mg/kg/dose
Children 20 kg and over and adults: 2.4 mg/kg/dose
- One dose on admission (H0)
- One dose 12 hours after admission (H12)
- One dose 24 hours after admission (H24)
- Then one dose once daily
Treat parenterally for at least 24 hours (3 doses), then, if the patient can tolerate the oral route, change to a complete 3-day course of an ACT. If not, continue parenteral treatment once daily until the patient can change to oral route (without exceeding 7 days of parenteral treatment).
If artesunate is not unavailable, artemether may be an alternative:
artemether IM into the anterior thigh (never administer by IV route)
Children and adults: 3.2 mg/kg on admission (D1) then 1.6 mg/kg once daily
Treat parenterally for at least 24 hours (2 doses), then, if the patient can tolerate the oral route, change to a complete 3-day course of an ACT. If not, continue parenteral treatment once daily until the patient can change to oral route (without exceeding 7 days of parenteral treatment).
Note: if patient is still on parenteral treatment on Day 5, continue on the same treatment until Day 7. In this case it is not necessary to start an ACT.
Quinine IV is still recommended in some national protocols. It may be used in treatment of malaria with shock if artesunate IV is not available. The dose is expressed in quinine salt:
- Loading dose: 20 mg/kg to be administered over 4 hours, then, keep the vein open with an infusion of 5% glucose over 4 hours; then
- Maintenance dose: 8 hours after the start of the loading dose, 10 mg/kg every 8 hours (alternate quinine over 4 hours and 5% glucose over 4 hours).
For adults, administer each dose of quinine in 250 ml of glucose. For children under 20 kg, administer each dose of quinine in a volume of 10 ml/kg of glucose.
Do not administer a loading dose to patients who have received oral quinine, or mefloquine within the previous 24 hours: start with maintenance dose.
Treat parenterally for at least 24 hours, then, if the patient can tolerate the oral route, change to a complete 3-day course of an ACT (or if not available, oral quinine to complete 7 days of quinine treatment). If not, continue parenteral treatment until the patient can change to oral route (without exceeding 7 days of parenteral treatment).
Symptomatic treatment and management of complications
Hydration
Maintain adequate hydration. As a guide, for volume to be administered per 24 hours by oral or IV route, see Appendix 1.
Adjust the volume according to clinical condition in order to avoid dehydration or fluid overload (risk of pulmonary oedema).
Fever
Paracetamol in the event of high fever only (Fever, Chapter 1).
Severe anaemia
For treatment, see Anaemia, Chapter 1.
Hypoglycaemia
For treatment, see Hypoglycaemia, Chapter 1.
Notes:
- In an unconscious or prostrated patient, in case of emergency or when venous access is unavailable or awaited, use granulated sugar by the sublingual route to correct hypoglycaemia e Citation e. Place a level teaspoon of sugar, moistened with a few drops of water, under the tongue, then place the patient in the recovery position. Repeat after 15 minutes if the patient has not regained consciousness. As with other methods for treating hypoglycaemia, maintain regular sugar intake, and monitor. .
- The risk of hypoglycaemia is higher in patients receiving IV quinine.
Coma
Check/ensure the airway is clear, measure blood glucose level and assess level of consciousness.
In the event of hypoglycaemia or if blood glucose level cannot be measured, administer glucose.
If the patient does not respond to administration of glucose, or if hypoglycaemia is not detected:
- Insert a urinary catheter; place the patient in the recovery position.
- Monitor vital signs, blood glucose level, level of consciousness, fluid balance (urine output and fluid input) hourly until stable, then every 4 hours.
- Rule out meningitis (lumbar puncture) or proceed directly to administration of an antibiotic (see Meningitis, Chapter 7).
- Reposition the patient every 2 hours; ensure eyes and mouth are kept clean and moist, etc.
Seizures
See Seizures, Chapter 1. Address possible causes (e.g. hypoglycaemia; fever in children).
Respiratory distress
- Rapid laboured breathing:
Check for pulmonary oedema (crepitations on auscultation), which may occur with or without fluid overload: reduce IV infusion rate if the patient is receiving IV therapy, nurse semi-sitting, oxygen, furosemide IV: 1 mg/kg in children, 40 mg in adults. Repeat after 1 to 2 hours if necessary.
Associated pneumonia should also be considered (see Acute pneumonia, Chapter 2).
- Slow, deep breathing (suspected metabolic acidosis):
Look for dehydration (and correct if present), decompensated anaemia (and transfuse if present).
Oliguria and acute renal failure
Look first for dehydration (Dehydration, Chapter 1), especially due to inadequate fluid intake or excessive fluid losses (high fever, vomiting, diarrhoea). Treat dehydration if present. Be aware of the risk of fluid overload and acute pulmonary oedema. Monitor for the return of urine output.
Acute renal failure (ARF) is found almost exclusively in adults and is more common in Asia than Africa. Insert a urinary catheter, measure output. Restrict fluids to 1 litre/day (30 ml/kg/day in children), plus additional volume equal to urine output. Renal dialysis is often necessary.
Antimalarial treatment in pregnant women
Uncomplicated P. vivax, P. ovale, P. malariae, P. knowlesi malaria
As other patients.
Primaquine should not be given in pregnancy.
Uncomplicated falciparum malaria
All ACT included in the table Treatment of uncomplicated falciparum malaria can be used in all trimesters.
If ACTs are not available, quinine PO (for dosing, see Treatment of uncomplicated falciparum malaria) combined with clindamycin PO if possible (10 mg/kg 2 times daily for 7 days) may be an alternative to ACT.
Primaquine should not be given in pregnancy.
Severe malaria
Artesunate, or if unavailable artemether, is recommended in all trimesters.
Quinine IV is not recommended as standard treatment, however it still remains in some national protocols.
Prevention
- For pregnant women in areas with high risk of infection with P. falciparum, refer to the guide Essential obstetric and newborn care, MSF.
- In areas with seasonal malaria transmission (in particular across the Sahel sub-region), seasonal malaria chemoprevention in children < 5 years reduces mortality: administer amodiaquine + SP at monthly intervals for 4 months during the transmission period
[4]
Citation
4.
World Health Organization. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. 2012.
https://www.who.int/malaria/publications/atoz/smc_policy_recommendation_en_032012.pdf?ua=1 . - In malaria endemic areas and in epidemic-prone contexts, all in-patient facilities (including HIV treatment centres and feeding centres), should be furnished with long-lasting insecticidal nets (LLINs). For more information, refer to the guide Public health engineering, MSF.
- See specialised literature for information regarding anti-vector measures and prevention in travellers.
- (a)Most rapid tests detect the following antigens alone or in combination: HRP2 protein specific to P. falciparum; an enzyme (Pf pLDH) specific to P. falciparum; an enzyme (pan pLDH) common to all 4 plasmodium species. HRP2 may continue to be detectable for 6 weeks or more after parasite clearance; pLDH remains detectable for several days (up to 2 weeks) after parasite clearance.
Use pan pLDH tests as first choice in hyper and holo-endemic areas, as well as in areas of intense seasonal transmission and during outbreaks or complex emergencies. In other contexts, HRP2 tests (P. falciparum > 95%) or HRP2 + pLDH combination tests (P. falciparum < 95%) are preferred. - (b) If the patient vomits within 30 minutes after administration: re-administer the full dose. If the patient vomits between 30 minutes and 1 hour after administration, re-administer half of the dose. If severe vomiting precludes oral therapy, manage as severe malaria, see Treatment of severe malaria.
- (c) ACT: a combination of artemisinin or one of its derivatives (e.g. artesunate, artemether) with another antimalarial of a different class.
- (d)If it is impossible to refer a patient to a center capable of providing parenteral treatment, rectal artesunate should be given according to the same schedule as artesunate slow IV injection (H0, H12, H24, then once daily).
- (e)Place a level teaspoon of sugar, moistened with a few drops of water, under the tongue, then place the patient in the recovery position. Repeat after 15 minutes if the patient has not regained consciousness. As with other methods for treating hypoglycaemia, maintain regular sugar intake, and monitor.
- 1.
World Health Organization. Guidelines for the treatment of malaria, 3rd ed. World Health Organization. 2015.
https://apps.who.int/iris/handle/10665/162441 - 2.World Health Organization. Compendium of WHO malaria guidance: prevention, diagnosis, treatment, surveillance and elimination. 2019.
https://apps.who.int/iris/handle/10665/312082 - 3.World Health Organization. WHO policy brief on single-dose primaquine as gametocytocide in Plasmodium falciparum malaria. 2015.
https://www.who.int/malaria/publications/atoz/who_htm_gmp_2015.1.pdf?ua=1 - 4.World Health Organization. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. 2012.
https://www.who.int/malaria/publications/atoz/smc_policy_recommendation_en_032012.pdf?ua=1