3.1 Cholera case register
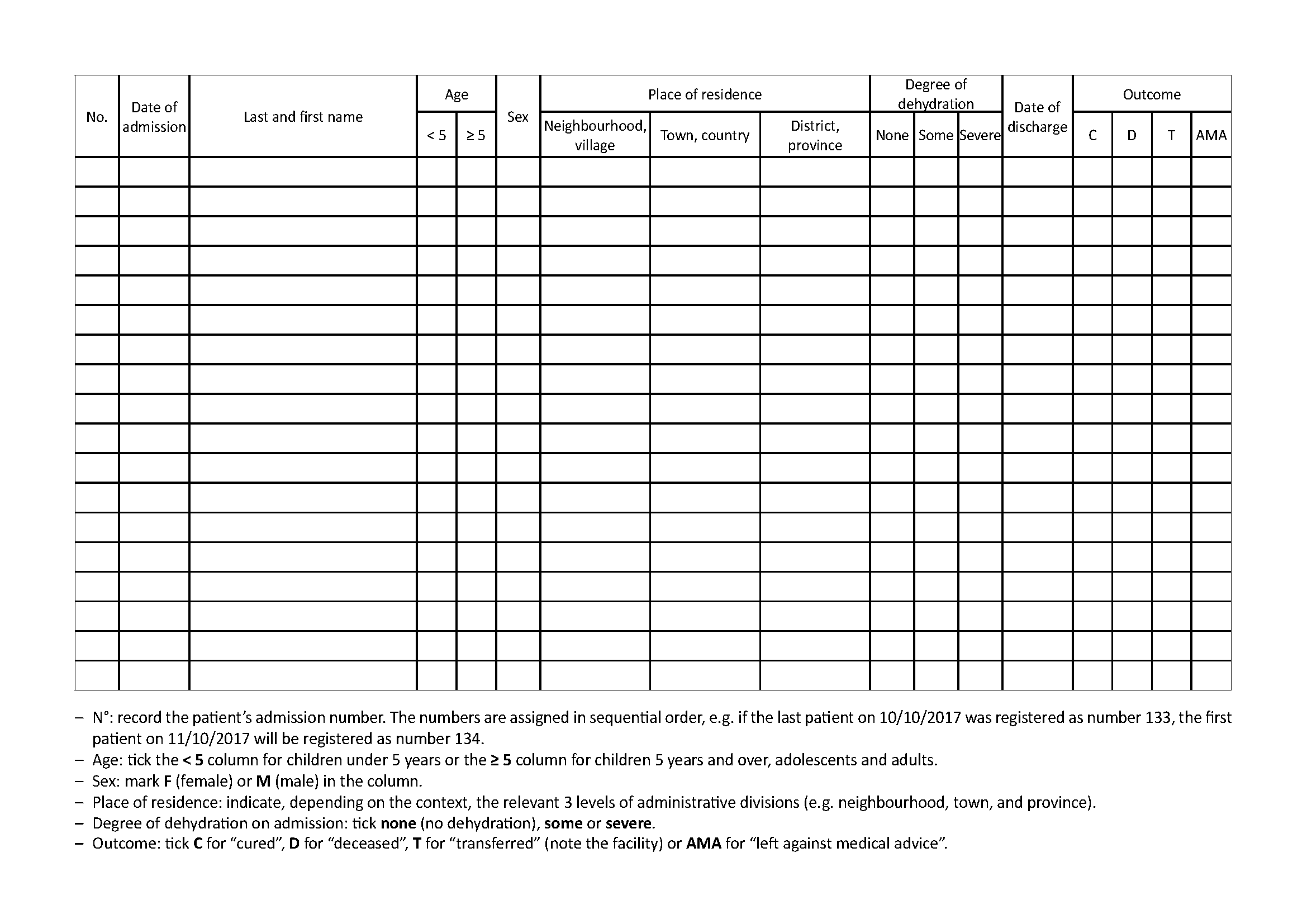
See Toolbox, Cholera case register
All cholera treatment facilities (CTCs, CTUs, and ORPs) must keep a register of cholera cases. This register must at least include:
- On the cover:
- Type and location of facility (e.g. CTC, Bel-o-Kan)
- Start and end date of the register (from dd/mm/yyyy to dd/mm/yyyy)
- On each page of the register:
- Admission number
- Patient’s identity (last name, first name, age, sex)
- Address or place of residence
- Date (dd/mm/yyyy) of admission and discharge (time of discharge if the patient has stayed only a few hours)
- Degree of dehydration on admission (none/some/severe)
- Outcome (cured/deceased/transferred (indicate the facility)/left against medical advice)
All patients hospitalised, kept under observation, or treated as outpatients (i.e. given sachets of ORS to take at home) must be entered into the register.
In the event of diagnostic error, a patient registered as a cholera case is not crossed off the register even if in the end they present another pathology unrelated to cholera.
Depending on the context or the Ministry of Health’s instructions, other information may be noted: length of illness, pregnancy status, previous vaccination against cholera, etc.
3.2 Patient file
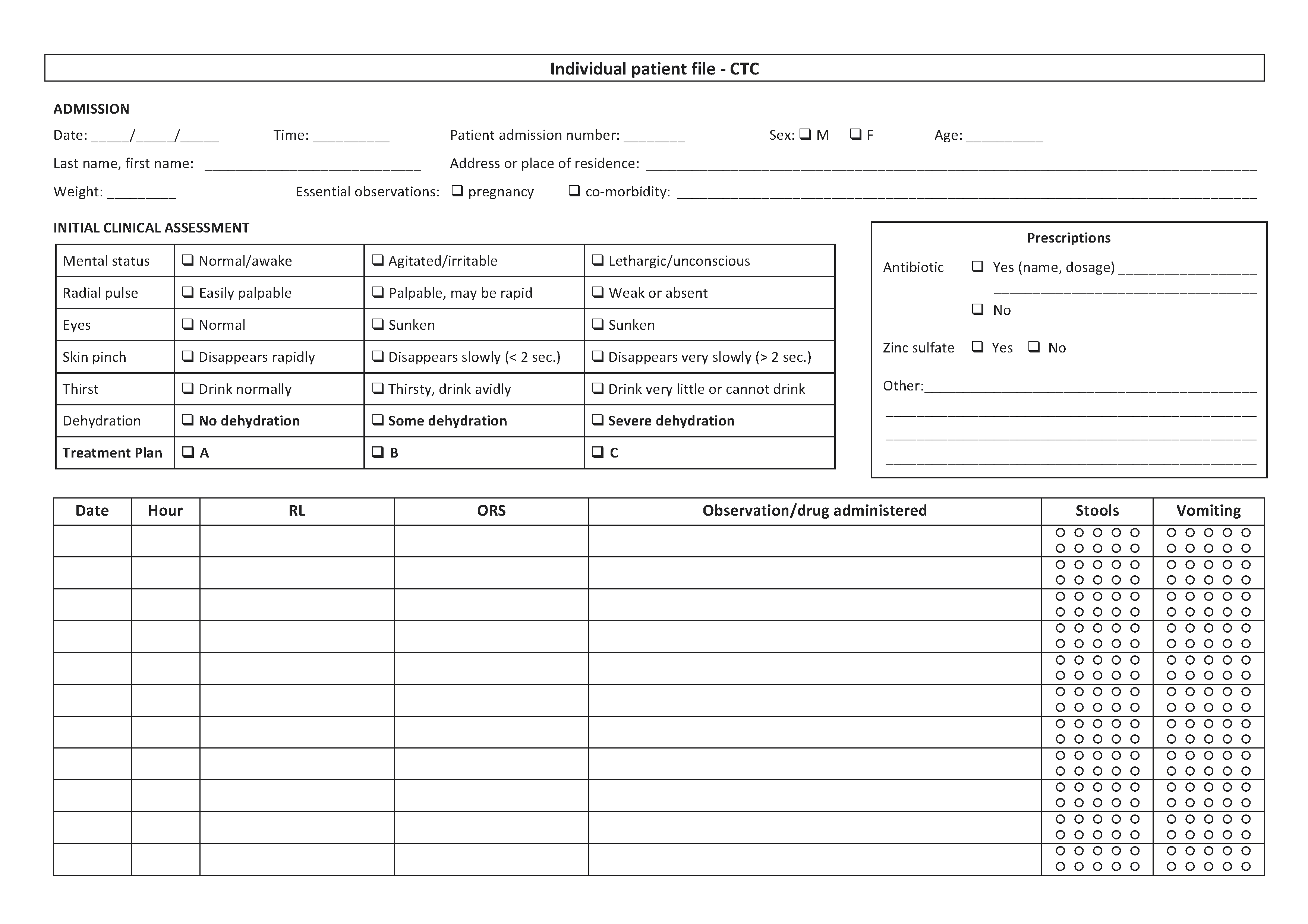
See Toolbox, Individual patient file
An individual patient file must be opened for every patient admitted to a cholera treatment facility. It contains:
- Patient admission number
- Patient’s identity: last name, first name, age, sex, address or place of residence
- Date of admission
- Clinical status on admission:
- Level of dehydration on admission (none/some/severe)
- Essential observations (weight if children under 5 years, pregnancy, known acute malnutrition, co-morbidity)
- Prescriptions:
- Rehydration protocol on admission (e.g. 4 litres of ORS over 4 hours)
- Other prescriptions (antibiotics, zinc, etc.)
- Surveillance:
- Clinical observation: mental status, pulse, etc.; blood pressure for pregnant women
- Details of fluid given (RL and/or ORS) and fluid lost (stools, vomiting)
- Date and discharge status (cured/deceased/transferred/leaving against medical advice)
- In the event of death, probable cause of death
The file should always be visible (e.g. hung above the patient) and must accompany the patient if s/he changes sector in the CTC/CTU.
Surveillance data and treatments administered are recorded as and when carried out.
If a patient has various files, the patient’s name must be marked on each file and the files stapled together.
The coordinator collects the files of discharged patients every day and archives them when all the data are compiled.
3.3 Water and sanitation register
CTCs and CTUs must set up a register monitoring water quality checks, the consumption of potable water, actions and materials used in relation with the production of potable water and chorine solutions.
This register includes:
- Quantity of water used per day in m3
- FRC in distributed water (twice per day)
- Quantity of NaDCC or calcium hypochlorite used per day to treat potable water and prepare chlorine solutions
- Quantity of chlorine solutions (0.05%, 0.2%, 2%) prepared per day
- Dates of bladder and distribution system inspections (once a month)
- Dates and results of chlorine quality controls (Wata test®)
3.4 Stock cards
CTCs, CTUs and ORPs should have stock cards for medical and non-medical materials (one card per article). These cards help to prepare orders and monitor consumption.
Note: all these documents are used to collect data for surveillance and evaluation purposes (Chapter 8).