Organization and rigorous management of the pharmacy are crucial in all health facilities in order to:
- maintain a permanent stock of essential medicines and supplies of quality;
- reduce costs;
- save time and optimise the work of the staff;
- facilitate management and continuous consumption evaluation.
In any case, national pharmaceutical policies and regulations must be taken into account when implementing pharmaceutical activities.
Preliminary information
Drug designation
All active ingredients have an international nonproprietary name (INN). Drugs are designated by their INN in all standardised lists. The INN should also be used in standard therapeutic regimens and management documents, in order to avoid confusion, since drugs are sold under their INN or a variety of brand names, depending on the manufacturer (e.g. ampicillin may be sold as Britapen®, Penbritin®, Pentrexyl®, Totapen®, etc.).
Generic drugs are copies of drugs whose patents have expired. They can therefore be made by any pharmaceutical laboratory and are most often sold under their INN or occasionally under a new brand name.
Selection of essential medicines
Most countries have a national list of essential medicines. If there is no national list, refer to the latest WHO list.
The use of such a list presents several advantages:
- it simplifies supply and reduces costs: most drugs on the WHO list are available in generic forms at affordable prices;
- it facilitates co-ordination of international aid and obtains approval from organizations which subsidise projects (United Nations, European Union, etc.).
The list of selected drugs is drawn in accordance with pre-established standardised therapeutic regimens. This offers two major advantages:
- better treatments due to more rational use of a restricted number of essential drugs;
- economic and administrative improvements concerning purchasing, storage, distribution and control.
Proposing the same drug in many different strengths or forms should be avoided. In most cases, one form/strength for adults and one paediatric form/strength are sufficient. This facilitates management and avoids confusion in prescriptions.
At times, local prescription usages should be taken into account, e.g. in French-speaking Africa, 500 mg aspirin tablets are used; in English-speaking Africa, 300 mg tablets.
Note: medical supplies (dressing, injections, sutures, etc.) should be limited to essentials and the object of a standardised list.
Drug classification
In the WHO list, drugs are classified according to their therapeutic action. This classification presents a certain pedagogical advantage but cannot be used as the basis of a storage arrangement system (e.g. a drug may appear in several classes).
Médecins Sans Frontières recommends a storage arrangement system according to the route of administration and in alphabetical order.
Drugs are divided into 6 classes and listed in alphabetical order within each class:
- oral drugs
- injectable drugs
- infusion fluids
- vaccines, immunoglobulins and antisera
- drugs for external use and antiseptics
- disinfectants
This classification should be used at every level of a management system (order forms, stock cards, inventory lists, etc.) in order to facilitate all procedures.
Levels of use
More limited lists should be established according to the level of health structures and competencies of prescribers. Restricted lists and the designation of prescription and distribution levels should be adapted to the terminology and context of each country.
Quantitative evaluation of needs when launching a programme
Once standard therapeutic regimens and lists of drugs and supplies have been established, it is possible to calculate the respective quantities of each product needed from the expected number of patients and from a breakdown of diseases.
Several methods have been suggested (see Estimating drug requirements, WHO). Quantities calculated may differ from those corresponding to true needs or demands (this can be the case when the number of consultations increases or when prescribers do not respect proposed therapeutic regimens).
In an emergency situation (especially with displaced population), the Emergency Health Kit, developed in collaboration with the WHO, UNHCR, MSF, etc., is designed to meet the care needs of a displaced population of 10,000 people for 3 months. Afterwards, specific local needs should be evaluated in order to establish a suitable supply.
Routine evaluation of needs and consumption allows verification of how well prescription schemes are respected and prevents possible stock shortages.
Layout of a pharmacy
Whether constructing a building, converting an existing building, central pharmacy or health facility pharmacy, the objectives are the same only the means differ.
Premises
Functional premises should be designed in order to ensure:
- the safe keeping of stocks;
- correct storage of drugs and supplies;
- rational and easy management.
Characteristics of a warehouse
Dimensions of warehouse are determined by storage needs, which depend on:
- the number of drugs and supplies to be stocked;
- the number and activities of facilities;
- distribution and receiving frequency: the lesser the frequency the greater the volume needed, thus the greater the space needed.
It is better to have too much space than not enough: a cramped warehouse is difficult to work, and any increases in stock or activity are also difficult. For 1 m2 of storage space count 3 m2 of floor space.
Security of stocks requires solid doors, locks, windows and ceilings.
Correct preservation of drugs depends on temperatures and humidity, conditions that are very often difficult to control in tropical countries.
- Correct ventilation is necessary; fans mainly reduce humidity, air-conditioning reduces heat and humidity.
- A ceiling underneath the roof is essential in order to reduce the ambient temperature; the space between the ceiling and roof must be ventilated.
- Windows and openings should be shaded to avoid exposure of drugs to direct sunlight.
- Floors should be covered in cement (slightly inclined, if possible, to facilitate cleaning).
Interior layout of a warehouse
The organization should be logical and correspond to the circuit "reception, storage, distribution".
Shelves and pallets
Solid and stable shelves are indispensable. In tropical countries where termites attack wood, metal structures are preferred. As they can be dismantled, it is easy to adjust spaces between shelves and alleys to better accommodate goods to be stored.
Space between shelves and walls improves ventilation.
No products or packaging, even large-sized, should be stored on the floor, but on pallets which permit air circulation and protect against humidity.
Stocking areas
Within a warehouse, or close by, stocking areas should be provided.
- Receiving area: for stocking parcels before unpacking and checking freight and quality control.
- Distribution area: for stocking peripheral orders before distribution. Each destination should have a designated area where parcels may be stocked before distribution.
Receiving and distribution areas should be near access doors in order to facilitate handling.
It is also recommended to plan a stocking area for empty boxes, used to prepare orders for peripheral health facilities.
Workspace(s)
A workspace should be set up in the receiving area and in the distribution area to verify deliveries and prepare orders.
Desk
For the person in charge of the pharmacy, a desk near a light source should be set up for administrative work and for keeping documents.
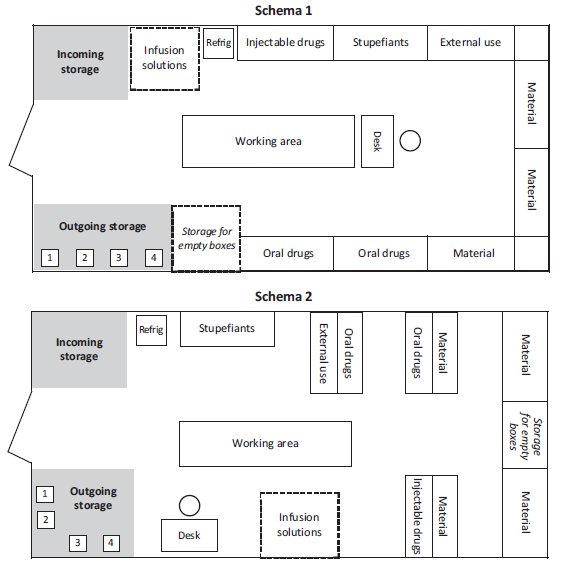
Examples of pharmacy layout
The arrangement of shelves, tables or other furniture, varies according to the layout of the premises.
For larger stocks or central pharmacies, use several rooms and apply the same principles by adapting layouts to needs: administration, cold room, refrigerators, etc.
Arrangement of drugs and supplies
Storage of drugs not requiring a cold chain
Drugs are arranged according to the classification adopted:
- oral drugs
- injectable drugs
- infusions
- drugs for external use and antiseptics
- disinfectants
In each category of products (oral, injectable, etc.) are classified alphabetically.
Each product should have a designated place, well identified by a fixed label indicating the INN, form and strength. By attributing a specific place to each item it is possible to immediately see the quantity available and to react quickly to avoid stock shortages.
Provide for sufficient space between and for each product.
Clearly indicate expiry dates on boxes (large marker). Arrange products with the earliest expiry date at the front of the shelves and those with the latest at the back. This is essential to avoid drugs expiring during storage.
So that persons not familiar with the INN system can find their way around in case of emergency or replacement, a list of commercial names and the corresponding INN can be put up, e.g.:
Bactrim® see co-trimoxazole
Clamoxyl® see amoxicillin
Flagyl® see metronidazole
Valium® see diazepam
Storage of controlled substances
Narcotics and other controlled substances should be placed under lock and key.
Storage of products requiring a cold chain
Products needing a cold chain should be stored in a refrigerator (between 2-8 °C): vaccines, immunoglobulins, serums, insulin, ergometrine, oxytocin, dinoprostone, certain laboratory tests, etc.
Storing medical materials/supplies
Given the diversity of items, do not to use alphabetical ordering, but group articles by category: injections, dressings, sutures, reagents and laboratory material, etc.
Storing bulky materials
Put a few boxes in their normal place and, on a label, indicate where the rest of the stock is kept. Do not disperse the rest of the stock in several places.
|
Management of a pharmacy
Organization of activities
The management of the pharmacy should be entrusted to a single person having received adequate training. This person is the only person possessing keys to the pharmacy and narcotics cupboard and is helped by one or more assistants, depending on the workload.
Tasks and responsibilities should be clearly defined. One assistant should be able to replace the person in charge if necessary.
It is important to draw up a work calendar (orders, distributions, inventories, management of expired drugs, etc.) in order to spread out the workload.
Stock management
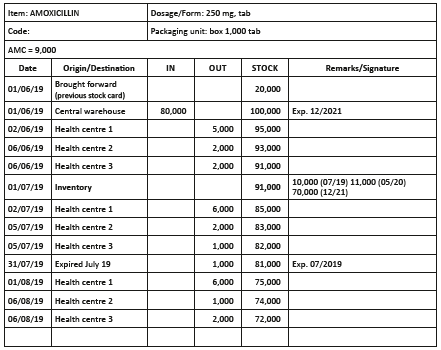
Stock cards
The stock card is the principle instrument for stock control. A stock card is established for each product (drugs and supplies) and updated at each movement. Stock cards are used to:
- identify all stock movements: in and out;
- determine at any moment the theoretical level of stocks;
- follow–up the consumption of different facilities;
- correctly plan and prepare orders;
- determine losses (differences between theoretical stock and actual stock).
Example of a stock card
The following should be noted on stock cards:
- the INN, form and strength;
- all movements (in, out, origin, destination, loss due to expiration, damages) and dates;
- inventories and dates.
The following may also be included:
- average monthly consumption;
- stock levels: buffer stock, running stock;
- other stock areas for a product;
- unit price;
- current orders and dates.
Quantities in and out are always recorded in units (e.g. 5,000 tablets, 80 ampoules) and never in number of boxes.
Write a single operation per line, even if several operations take place the same day.
Note: stock cards are always equired, even when computer assisted stock management is used.
Quantities to retain and order (stock level)
Average monthly consumption (AMC)
Calculated from outgoing stock recorded on stock cards: add the quantities of several months (3, 6 or 12) in the out column and divide the total by the number of months considered.
Running stock = consumption between two supply deliveries
Running stock corresponds to the quantity of each drug consumed between two supply deliveries (e.g. if deliveries are quarterly, running stock = AMC x 3).
Buffer stock
This stock is planned to compensate for possible late deliveries, losses, and increases in consumption. It is calculated according to the delivery delay of orders.
Buffer stock quantities are generally evaluated as half of the consumption during the period between two deliveries. It depends on risks that a programme may run: stock shortages or drug expiration in specific situations (resources, seasonal supply problems, etc.).
For example, if the delivery delay is two months, the buffer stock corresponds to the quantity consumed in one month.
Quantities to be ordered
Quantities to order are based on data from stock cards:
- actual stock level (inventory) on the day of the order
- running stock
- buffer stock
- delay period between order and delivery
- orders not yet delivered
Order = (running stock + buffer stock + probable consumption during delivery delay) – (inventory + orders not yet delivered).
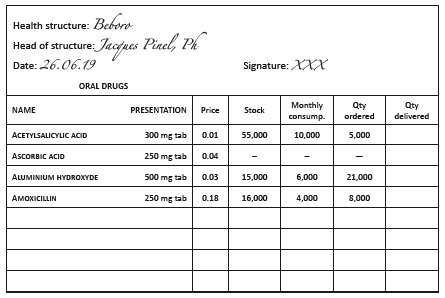
Order and delivery forms
Concerning orders from peripheral facilities to the central pharmacy, it is recommended to use pre-printed order forms which indicate the INN, form (tablet, capsule, vial, ampoule, etc.) and strength.
The following may also be included:
- stock levels,
- AMC.
Orders should be in triplicate, dated and countersigned by the person in charge of the health facility. Two copies are sent to the central pharmacy: one serves as a way bill and may also be used for invoicing, the second stays with the central pharmacy. The third copy stays at the health facility.
Example:
Health facility order form, 6-month supply period, minimum stock of 3 months (2 month delivery delay + 1 month buffer stock )
Receiving orders
All orders should be accompanied by a way bill or invoice and packing list.
On reception, the number of parcels should be checked, then their contents should be verified:
- ensure that products delivered correspond to products ordered, and that the quantities conform to those on the packing list;
- packaging, labelling and expiry dates of each product should be checked, as well as the aspect of the product;
- look for special storage conditions (cold chain).
The supplier should be notified of all irregularities.
Then, drugs and material are integrated into stocks at their designated places. Incoming quantities are recorded on stock cards.
Way bills, invoices and packing lists are to be classed with orders in an "orders" file and kept for 3 years or more according to current regulations.
Inventory
An inventory of current stock quantities and expiry dates should be done before each order.
Stock cards give a theoretical figure of stock quantities, but actual quantities of each product should be verified (physical stock). Differences may arise due to errors in recording or due theft. These differences should be clarified.
An inventory may only be easily done if the pharmacy is correctly arranged. It is an indispensable task.
During an inventory there should be no stock movements, i.e. incoming or outgoing stock.
Distribution
Distribution to health facilities
Each health facility sends the central pharmacy two copies of the order form.
On both copies, actual quantities supplied by the central pharmacy are recorded in the “Qty delivered” column.
One on these copies is sent with the delivery.
After verifying that all products have been correctly recorded on their respective stock cards, the second copy is placed in a file established for health facility. The exit date on the stock card should be the same as the date on the order form.
Dispensing drugs to patients
Drug packaging should be presentable. Use plastic bags that can be resealed by pressure (Minigrip®).
Prepare labels for each drug, clearly showing:
- the name of the drug (INN), form and strength;
- the dosage written out in full or in symbols.
Put the number of tablets corresponding to a complete treatment and the label into the bag.
In busy centres it is better to have two people responsible for dispensing drugs in order to double check prescription deliveries; the first collects the drugs prescribed, the second verifies and gives them to patients with all necessary explanations, slightly away from other patients.
So that patients correctly follow treatment, adequate explanations should be given:
- how to take the drug,
- for how long,
- possible adverse effects (e.g. drowsiness caused by anti-histamines),
- precautions to be taken (e.g. avoid alcohol with metronidazole).
Persons dispensing drugs should be able to give patients the information they need.
Interpreters are needed if several languages exist in the same region.
Donations of recuperated medicines and medical samples
It is not recommended to solicit or accept supplies coming from collections of drugs recuperated from consumers in industrialised countries, or free samples distributed by manufacturers.
They are very often specialised drugs unknown to prescribers and unsuitable for local pathologies. The multiplication of different drugs supplied interfere with the implementation of standardised therapeutic regimens and makes any form of management impossible.