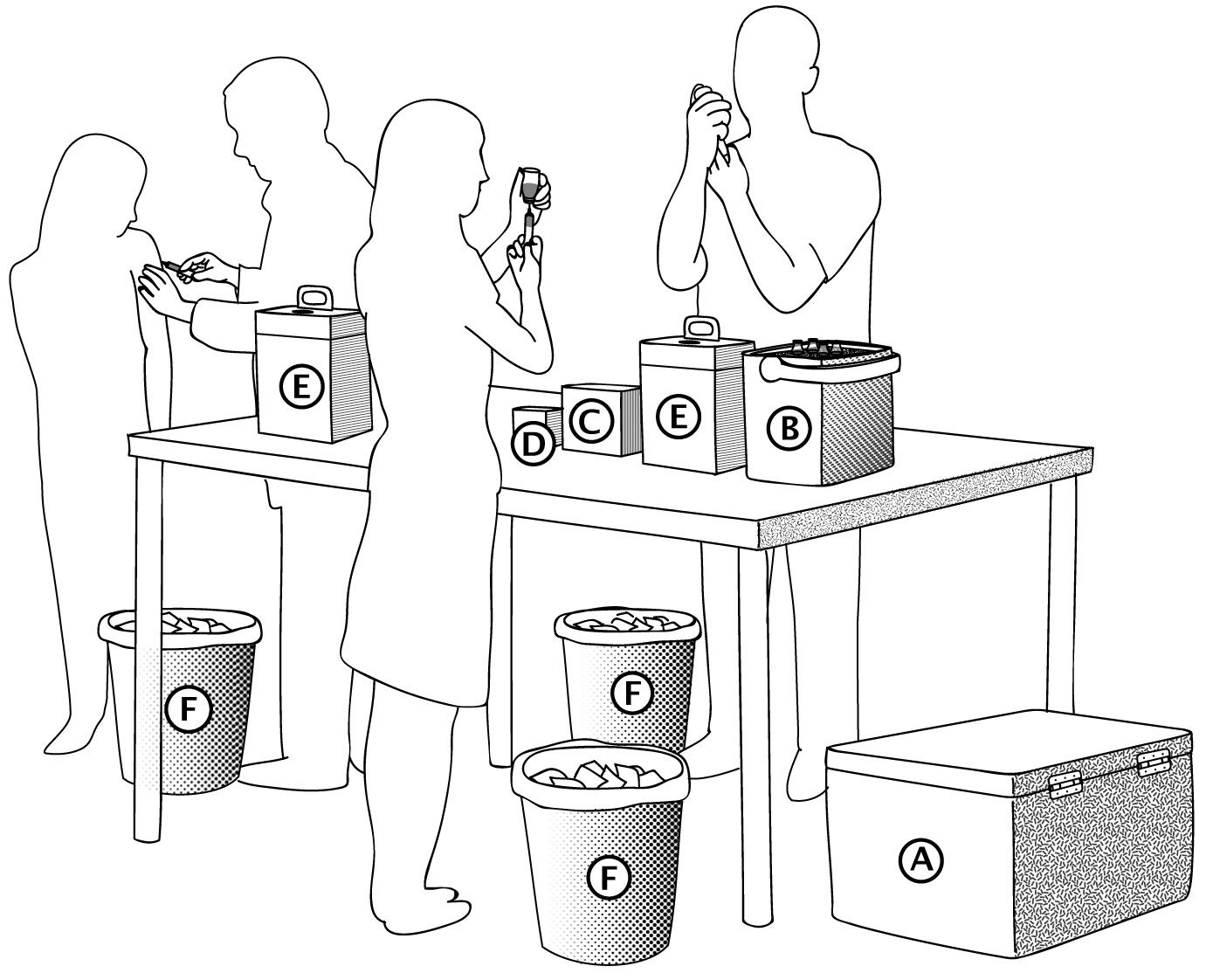
See Toolbox
The recommended methods for vaccination campaigns are different than those for routine vaccination. Staff should receive specific training prior to the campaign.
34.1 Quality of care criteria
- Aseptic technique is used when reconstituting vaccine and preparing syringes.
- The dose prepared in the auto-disable syringe (ADS) is the correct dose for administration.
- The temperature and time limits (6 hours) for reconstituted vaccine storage are respected.
- Sharps are collected and safely transported in special safety boxes (sharps containers).
34.2 Supplies/equipment needed for vaccine preparation
- Vials of vaccine (lyophilised powder) and vials/ampoules of diluent
- Hand hygiene supplies (soap, bowl, hand towel and water) or alcohol-based solution
- Pliers or scissors for removing the protective cap from vials or a file for ampoules
- Sterile 5-, 10- or 20-ml syringes (depending on the volume of diluent) and sterile 19g (cream colour) needles for reconstituting the vaccine
- Sterile 0.5-ml ADS
- Clean tray
- Safety boxes for collection, transport and disposal of sharps
- Cotton wool
34.3 Cold chain for storing vaccines
Vaccines must be kept between +2 and +8 °C throughout the entire chain (storage at the site and holding vials after reconstitution).
The equipment for the site varies with the number of teams aCitation a.In densely-populated areas, a vaccination site can accommodate one or two teams, at most. With more than two teams, the crowd is too large. It is better to open second site. Ideally, two teams per site rationalises the logistical resources and supervision. :
| 1 vaccination team | 2 vaccination teams |
|---|---|
|
1 RCW25 Electrolux® cold box + thermometer |
1 RCW25 Electrolux® cold box + thermometer |
The cold box can be used to store vaccines and diluent for one or two teams at one site. The volume per dose (vaccine and diluent) varies, depending on manufacturers. for example, if the volume is 3 cm3/dose, 3,000 doses of vaccine and diluent can be stored on each site. Check before the beginning of vaccination session.
To minimise the risk of freezing or breakage:
- Leave the ice packs at room temperature for at least 30 minutes before placing them in the cold box. The ice packs are ready when the frost on the outside has melted and the ice inside has begun to melt (there is a little water inside the pack).
- Leave the vaccine vials in their box so that they do not come in direct contact with the ice packs.
Monitor the temperature (thermometer/VVM).
The vaccine carrier (one per team) is used by the two preparers.
It is used for intermediate storage. Small amounts of vaccine and diluent are taken out of the cold box as needed to limit how often the cold box is opened.
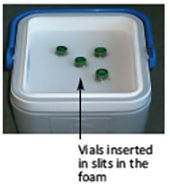
Vials of reconstituted vaccine are placed in the slits in the foam pad in the vaccine carrier lid.
See the illustration below.
|
|
A. Cold box |
The number of ice packs
bCitation b.Note: sometimes the number of ice packs is less than that recommended by the manufacturer, but enough to keep the vaccine at the recommended temperature (as long as there is still ice in the ice packs). This reduces the number of ice packs needed.
in each cold box and vaccine carrier will depend on the outside temperature:
|
|
Electrolux® RCW25 cold box |
Giostyle® vaccine carrier |
|---|---|---|
|
Ice packs |
0.6-litre |
0.4-litre |
|
If outside |
12 to store the vaccines for 2 days |
6 per vaccine carrier, |
|
If outside |
18 to store the vaccines for 2 days |
8 per vaccine carrier, |
- Cold boxes and vaccine carriers should be clean and dry.
- To reduce the risk of the vaccines freezing, ice packs should be left out for at least 30 minutes at room temperature until the ice begins to melt (water forms; check by shaking them) before being placed into cold boxes.
- Wipe and dry the ice packs before placing them in cold boxes and vaccine carriers.
- keep the vaccine vials in their box or in a plastic bag to prevent the labels from peeling off (from the moisture).
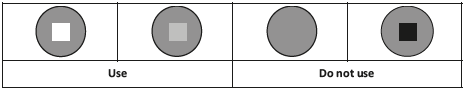
The vaccine vial monitor affixed to each vaccine vial allows verification that the vaccine has not been damaged by heat. When the vial is exposed to heat, the square inside the circle darkens. Only use vials whose squares are lighter than the surrounding circles:
34.4 Reconstituting the vaccine
- Wash hands or disinfect them with an alcohol-based solution. Preparers do not need to wear gloves.
- Take one vial of vaccine and one vial of diluent from the vaccine carrier.
- Check:
- the name of the vaccine;
- the name of the diluent, and that each diluent corresponds to a vaccine (same manufacturer);
- the expiry date;
- the appearance of the lyophilised powder and the diluent (colour and clarity);
- the vaccine vial monitor (VVM).
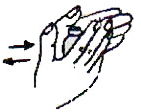
- Tap the vial or ampoule of vaccine so that the powder settles.
1. Open the vial or ampoule:
for a vial, remove the protective cap:
- pre-scored metal cap: remove with pliers;
- plastic cap: pop it off with thumb.
For an ampoule, carefully break off the end while holding the ampoule with clean cotton wool.
2. Fit the 19G needle onto the syringe.
Withdraw the recommended amount of diluent (see the package insert).
Insert the needle into the vaccine vial and gently inject the diluent. Remove the syringe and needle together and discard them in the sharps container without recapping the needle.
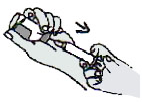
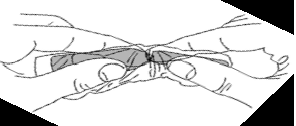
3. Roll the vial between the palms to thoroughly dissolve the powder.
Check the appearance (colour and clarity) and make sure there are no crystals. If in doubt, do not administer the vaccine and consult the appropriate person.
4. Store the reconstituted vaccine in the slits in the vaccine carrier’s foam pad (the vaccine is heat-sensitive).
Each preparer reconstitutes only one vial at a time and then fills the syringes.
Do not reconstitute a large number of vaccine vials that may not be used.
Without replacing the ice packs, vaccine carriers left open for 8 hours at an average ambient temperature:
- of 25 °C will maintain an average internal temperature of 4 °C;
- of 31 °C will maintain an average internal temperature of 7 °C.
Summary
- When using a lyophilised vaccine for the first time: read the package insert.
- Diluents are not interchangeable. Each manufacturer supplies a specific diluent (composition) for each type of lyophilised vaccine.
- Diluents must be refrigerated for at least 12 hours before reconstitution so that they are at the same temperature as the vaccine at the time of preparation (between +2 and +8 °C).
- All reconstituted vaccine should be stored between +2 and +8 °C and discarded after 6 hours.
- use one reconstitution syringe and needle per vial. Do not re-use them to reconstitute other vials.
- In case of an accidental cut when opening an ampoule, there is a risk of vaccine contamination. Discard the ampoule, cover the wound with a dressing and put on gloves.
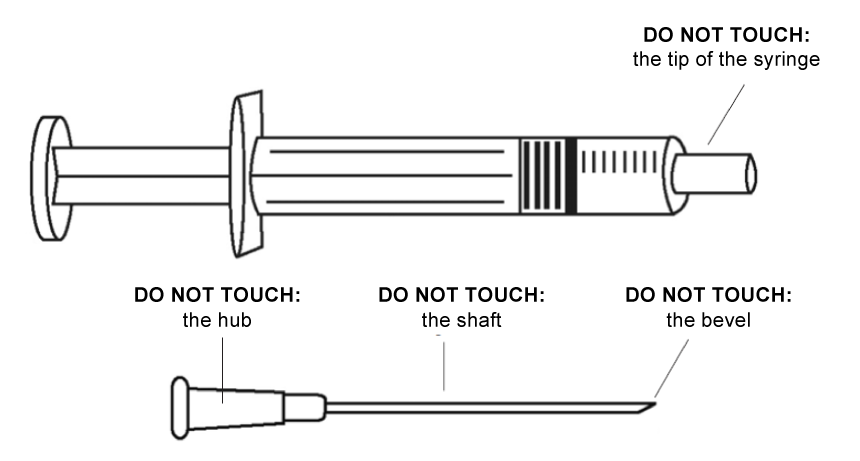
- Do not touch the needle or the end of the syringe.
- Never re-cap needles.
34.5 Preparing auto-disable syringes (ADS) for vaccine administration
- Do not remove syringes from their packaging in advance.
- Stick the ADS needle perpendicularly into the vial stopper.
- Invert the vial and hold it straight up and down.
- keep the point of the needle below the fluid level of the vaccine.
- Draw exactly 0.5 ml into the ADS.
- Remove the ADS from the vial.
- Purge any air by tapping the ADS, holding it vertically with the needle pointing up. A drop of vaccine should appear at the bevel of the needle.
- Check to make sure that the ADS contains 0.5 ml of vaccine. Do not use an ADS containing less than 0.5 ml.
Note: a 10-dose vial holds enough to fill ten 0.5 ml ADS. If the last syringe is not completely filled, add vaccine from another vial.
In case of accidental needle stick when handling the prepared ADS: DO NOT USE, discard immediately in the safety box.
34.6 Using prepared syringes
Give the prepared ADS directly to the vaccinator.
Prepare the syringes a few at a time, depending on the flow of people to vaccinate.
When the flow is heavy (e.g., urban areas, IDP camps or schools) or early in the day, syringes are prepared at a sustained pace. Good coordination between the preparers and the vaccinator is needed to prevent mishaps.
Vials containing leftover doses at the end of a session are collected and destroyed.
All vaccine and diluent vials are collected and counted for monitoring.
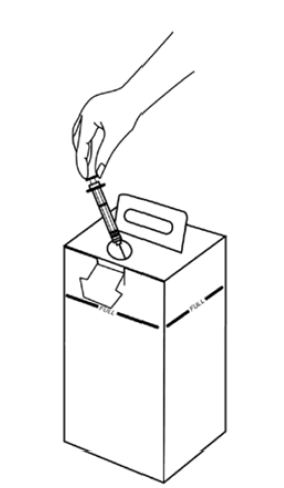
34.7 Using the safety box
- All used sharps are discarded in a safety box immediately after use.
- If possible, use 15-litre safety boxes for vaccination campaigns cCitation c.There are 5-, 10- and 15-litre safety boxes that can hold 100, 200 and 400 syringes, respectively. .
- Do not go beyond the safety box’s maximum syringe capacity. Do not fill beyond the maximum line shown.
- Do not handle the safety boxes unnecessarily, shake them, or compress them.
- Store them in a safe place, out of reach of the public, while they wait to be transported for disposal.
- The personnel that handle the safety boxes should always wear thick gloves (at the vaccination site, during transport to the disposal site and at the disposal site).
- They should never be carried in someone’s arms.
- (a)In densely-populated areas, a vaccination site can accommodate one or two teams, at most. With more than two teams, the crowd is too large. It is better to open second site. Ideally, two teams per site rationalises the logistical resources and supervision.
- (b)Note: sometimes the number of ice packs is less than that recommended by the manufacturer, but enough to keep the vaccine at the recommended temperature (as long as there is still ice in the ice packs). This reduces the number of ice packs needed.
- (c)There are 5-, 10- and 15-litre safety boxes that can hold 100, 200 and 400 syringes, respectively.